DiGeorge Syndrome: Understanding a Complex Genetic Disorder
Introduction
DiGeorge syndrome (DGS) is a congenital disorder caused by a deletion of a small segment of chromosome 22, specifically at the 22q11.2 locus. This genetic condition can lead to a wide range of health issues, including heart defects, immune deficiencies, and developmental delays. Due to its variable presentation, DGS often poses challenges in diagnosis and management. This article will explore the historical background, anatomy and pathophysiology, causes, symptoms and clinical presentation, diagnosis, treatment options, prognosis, living with DiGeorge syndrome, ongoing research, and future directions related to this condition.
What is DiGeorge Syndrome?
DiGeorge syndrome is characterized by the absence or underdevelopment of several body systems due to the deletion of genes on chromosome 22. The classic triad of symptoms includes congenital heart defects, hypoparathyroidism leading to hypocalcemia, and thymic hypoplasia resulting in immunodeficiency. However, the manifestations of DGS can vary widely among individuals. Symptoms may range from mild learning disabilities to severe health complications that require extensive medical intervention.
Historical Background
The history of DiGeorge syndrome dates back to the early 1960s when Dr. Angelo DiGeorge first described a group of patients with similar clinical features. The condition was initially recognized as a combination of immunodeficiency and congenital anomalies. Over the years, advances in genetic testing have allowed for better identification of the chromosomal deletion associated with DGS. The understanding of this syndrome has evolved significantly, leading to improved diagnostic criteria and treatment approaches.
Anatomy and Pathophysiology
To understand DiGeorge syndrome better, it is essential to examine the anatomy involved:
- Chromosome 22: The deletion in chromosome 22 at the 22q11.2 locus disrupts the development of structures derived from the pharyngeal pouches during embryogenesis.
- Thymus Gland: The thymus plays a crucial role in developing T-cells, which are essential for a functioning immune system. In individuals with DGS, thymic hypoplasia or aplasia leads to reduced T-cell production and increased susceptibility to infections.
- Parathyroid Glands: These glands regulate calcium levels in the body. Hypoparathyroidism due to parathyroid gland underdevelopment can result in low calcium levels (hypocalcemia), leading to various complications such as muscle spasms or seizures.
The pathophysiology of DGS involves multiple systems and highlights the interconnectedness of genetic factors and physical health.
Causes
DiGeorge syndrome is primarily caused by a deletion on chromosome 22:
- Genetic Deletion: Approximately 90% of cases result from a microdeletion at chromosome 22q11.2 that affects multiple genes critical for normal development.
- Inheritance Patterns: Most cases occur sporadically; however, about 10% are inherited from an affected parent in an autosomal dominant manner.
- Environmental Factors: While not directly causing DGS, certain environmental exposures during pregnancy (such as maternal diabetes or viral infections) may increase the risk of congenital anomalies.
Understanding these causes helps inform genetic counseling for families affected by DGS.
Symptoms and Clinical Presentation
Symptoms associated with DiGeorge syndrome can vary widely but commonly include:
- Congenital Heart Defects: These may include tetralogy of Fallot, ventricular septal defects (VSD), or interrupted aortic arch.
- Hypoparathyroidism: Low levels of parathyroid hormone lead to hypocalcemia, which can cause muscle cramps, seizures, or tetany.
- Thymic Hypoplasia: Reduced thymus size results in immunodeficiency; affected individuals are more susceptible to infections.
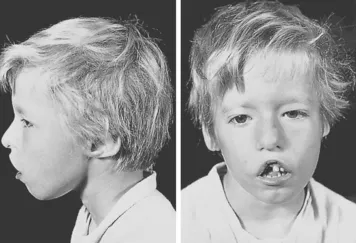
- Facial Features: Characteristic facial features may include low-set ears, wide-set eyes (hypertelorism), a small jaw (micrognathia), and cleft palate.
- Developmental Delays: Children with DGS may experience delays in speech and motor skills development.
- Psychiatric Disorders: There is an increased risk for mental health issues such as anxiety disorders and schizophrenia in adolescents and adults with DGS.
Recognizing these symptoms early can facilitate timely intervention and management strategies.
Diagnosis
Diagnosing DiGeorge syndrome typically involves several steps:
- Clinical Evaluation: A healthcare provider will assess physical characteristics and symptoms indicative of DGS.
- Medical History Review: Family history and any known congenital anomalies will be evaluated.
- Genetic Testing:
- Fluorescence in situ hybridization (FISH) testing can confirm the presence of a deletion at chromosome 22q11.2.
- Chromosomal microarray analysis may also be performed for more comprehensive genetic evaluation.
- Blood Tests:
- Serum calcium levels are measured to assess parathyroid function.
- Complete blood count (CBC) may help evaluate immune function by assessing T-cell counts.
- Imaging Studies:
- Echocardiograms are crucial for identifying congenital heart defects.
- Chest X-rays may help visualize thymic shadow or other structural abnormalities.
A comprehensive evaluation ensures accurate diagnosis and appropriate treatment planning.
Treatment Options
Treatment for DiGeorge syndrome focuses on managing symptoms and addressing specific complications:
- Multidisciplinary Care:
- A team approach involving pediatricians, cardiologists, immunologists, endocrinologists, speech therapists, and surgeons ensures comprehensive care tailored to individual needs.
- Surgical Interventions:
- Surgical repair may be necessary for congenital heart defects identified at diagnosis.
- Cleft palate repair can improve feeding and speech outcomes in affected children.
- Hormonal Replacement Therapy:
- Calcium supplementation along with vitamin D is often necessary to manage hypocalcemia due to parathyroid dysfunction.
- Regular monitoring of calcium levels is essential to prevent complications associated with hypoparathyroidism.
- Immunotherapy:
- In cases where T-cell function is severely impaired due to thymic hypoplasia, thymus transplantation or stem cell transplantation may be considered as potential treatment options.
- Supportive Therapies:
- Physical therapy can help improve motor skills and coordination.
- Speech therapy may be beneficial for addressing communication delays.
Effective management often requires a combination of treatments tailored to individual needs based on symptom severity and response to initial therapies.
Prognosis and Recovery
The prognosis for individuals with DiGeorge syndrome varies based on several factors:
- Early diagnosis and intervention significantly improve outcomes; many children can lead healthy lives with appropriate medical care.
- While some individuals experience significant physical challenges due to cardiac anomalies or immune deficiencies, others may have milder symptoms that do not severely impact daily functioning.
- Regular follow-up care is crucial for monitoring growth patterns and addressing any emerging complications promptly.
With proper management strategies in place, many individuals experience improved quality of life despite their challenges associated with DiGeorge syndrome.
Living with DiGeorge Syndrome
Living with DiGeorge syndrome requires ongoing management strategies:
- Education and Awareness: Understanding triggers and symptoms helps individuals cope better with their condition.
- Support Networks: Connecting with support groups or counseling services provides emotional support for those affected by this condition.
- Self-Care Strategies: Implementing lifestyle modifications—such as maintaining good nutrition—can help manage overall health during treatment.
- Open Communication with Healthcare Providers: Regular discussions about symptoms and treatment efficacy ensure optimal care throughout therapy courses involving ATRA or ATO treatments.
Encouraging open dialogue fosters trust between patients and healthcare providers while promoting proactive management strategies during treatment courses involving ATRA or ATO treatments.
Research and Future Directions
Ongoing research into DiGeorge syndrome aims to enhance understanding and improve treatment options:
- Investigating Genetic Factors: Researching genetic predispositions may lead to better-targeted therapies for those at risk for developing this condition during leukemia treatments.
- New Therapeutic Approaches: Studies are exploring novel medications that could offer more effective relief from symptoms associated with DiGeorge syndrome while minimizing side effects.
- Patient Education Initiatives: Developing educational programs aimed at increasing awareness about preventive measures will improve diagnosis rates and treatment outcomes among healthcare professionals involved in leukemia care.
These research efforts aim not only to improve care for existing patients but also enhance understanding for future generations affected by this condition.
Conclusion
DiGeorge syndrome is a complex genetic disorder that presents unique challenges for affected individuals and their families. By understanding its causes, symptoms, diagnosis methods, treatment options, and ongoing research efforts, we can improve awareness and outcomes for those living with this condition. With proper care from healthcare professionals throughout therapy courses involving ATRA or ATO treatments, many individuals can manage their symptoms effectively while maintaining a high quality of life post-recovery from this challenging condition.
Disclaimer: This article is intended for informational purposes only and should not be considered medical advice. Always consult a healthcare professional for diagnosis and treatment options tailored to your individual health needs.